Ozanimod Could Drive Celgene’s Long-Term Growth
The Phase 3 RADIANCE trial enrolled 1,313 RMS patients and conducted the trial in 21 countries.
Aug. 11 2017, Updated 7:36 a.m. ET
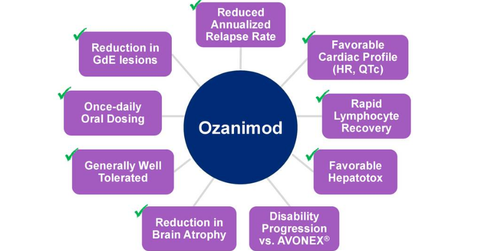
About Ozanimod
Ozanimod is a selective sphingosine 1-phosphate 1 (or S1PR1) and 5 (or S1PR5) receptor modulator in the investigation for its efficacy and safety in the treatment of relapsing multiple sclerosis, ulcerative colitis, and Crohn’s disease.
SUNBEAM and RADIANCE trial
In February 2017, Celgene (CELG) presented results from its Phase 3 SUNBEAM trial. This trial was conducted to evaluate the efficacy and safety of ozanimod for the treatment of individuals with relapsing multiple sclerosis (or RMS).
The Phase 3 SUNBEAM trial assessed the safety and efficacy of ozanimod 0.5 mg and ozanimod 1 mg compared to intramuscular interferon beta-1a (Avonex) for at least a 12-month therapy period. The study included 1,346 RMS patients and was based in 20 countries.
The data from the Phase 3 SUNBEAM trial demonstrate that both ozanimod 0.5 mg and ozanimod 1 mg therapy arms showed statistically substantial and clinically meaningful progress compared to Avonex for the primary endpoint of reducing annualized relapse rate (or ARR). The safety and tolerability data from the SUNBEAM trial remained consistent with the previously reported long-term results from the Phase 2 RADIANCE trial.
In May 2017, Celgene announced positive results from the Phase 3 RADIANCE trial. Celgene conducted the Phase 3 pivotal RADIANCE trial for evaluation of the safety and efficacy of oral ozanimod in individuals with RMS. The RADIANCE trial met its primary endpoint of reducing ARR compared to Avonex.
The Phase 3 RADIANCE trial enrolled 1,313 RMS patients and conducted the trial in 21 countries. The ozanimod 0.5mg and ozanimod 1 mg therapy demonstrated statistically substantial and clinically meaningful progress compared to Avonex.
Leading multiple sclerosis drugs include Sanofi’s (SNY) Aubagio; Biogen’s (BIIB) Fampyra, Tecfidera, and Tysabri; Novartis’s (NVS) Gilenya; Teva Pharmaceuticals’ Copaxone; and Merck’s Rebif. The iShares Core S&P 500 ETF (IVV) invests ~0.50% of its total portfolio holdings in Celgene.
