Keytruda’s Developments in June 2017
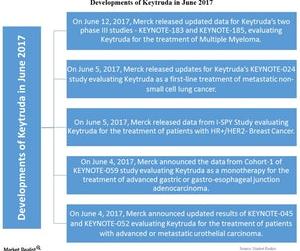
On June 12, 2017, Merck released updated data for Keytruda’s two phase III studies—KEYNOTE-183 and KEYNOTE-185—which evaluate Keytruda in combination with other drugs for the treatment of multiple myeloma.
July 4 2017, Updated 7:36 a.m. ET
Keytruda
As we discussed earlier, Keytruda (pembrolizumab) is a prescription medicine from Merck & Co.’s (MRK) Immuno-oncology franchise used for the treatment of non-small cell lung cancer, melanoma, squamous cell carcinoma of head and neck, classical Hodgkin lymphoma, advanced urothelial bladder cancer, and microsatellite instability–high cancer.
Developments
There were a number of key developments for Keytruda during June 2017.
On June 12, 2017, Merck released updated data for Keytruda’s two phase III studies—KEYNOTE-183 and KEYNOTE-185—which evaluate Keytruda in combination with other drugs for the treatment of multiple myeloma.
On June 5, Merck released updated data for Keytruda’s KEYNOTE-024 study, a phase III study that evaluates Keytruda as a first-line treatment of metastatic non-small cell lung cancer (or NSCLC) in patients with a high level of PD-L1. The updated data showed a continued overall survival benefit of Keytruda compared to chemotherapy.
On June 5, Merck released data from the I-SPY Study, a phase II study evaluating Keytruda in combination with standard Neoadjuvant therapy for the treatment of patients with HR+/HER2- breast cancer.
On June 4, 2017, Merck announced the data from Cohort-1 of KEYNOTE-059 study, a phase II study evaluating Keytruda as a monotherapy for the treatment of advanced gastric or gastroesophageal junction adenocarcinoma in patients who have received at least two lines of chemotherapy. The results show an overall response rate of 11.6% in such patients. Patients with PD-L1 reported higher response rates to the drug.
On June 4, Merck announced updated results of KEYNOTE-045, a phase III study, and KEYNOTE-052, a phase II study. Both studies evaluated Keytruda for the treatment of patients with advanced or metastatic urothelial carcinoma.
On June 3, 2017, Merck and Incyte (INCY) announced data from the ECHO-202 study, a phase I/II study evaluating the combination of Incyte’s Epacadostat and Merck’s Keytruda for the treatment of patients with advanced NSCLC, irrespective of the PD-L1.
On June 3, Merck released new data from two Phase II studies—KEYNOTE-158 and KEYNOTE-164—evaluating Keytruda as a monotherapy for advanced microsatellite instability-high or mismatch repair deficient solid tumors.
On June 3, Merck released updated results from Cohort G1 of KEYNOTE-021 study, a phase 1/2 study evaluating Keytruda in combination with pemetrexed and carboplatin, compared to only pemetrexed and carboplatin as a first-line treatment for advanced NSCLC.
On June 2, 2017, Merck released updated long-term overall survival data for Keytruda based on KEYNOTE-006, a phase III study that evaluated Keytruda for the treatment of metastatic melanoma.
To divest company-specific risk, investors can consider the VanEck Vectors Pharmaceuticals ETF (PPH), which holds 5.0% of its total assets in Merck. PPH also holds 5.1% in Pfizer (PFE), 5.0% in Novo Nordisk (NVO), and 4.9% in Sanofi (SNY).
