Delstrigo, Pifeltro, and Merck’s Antiviral Therapy Portfolio
Gilead Sciences’ Atripla, Genvoya, and Stribild generated revenues of $663.0 million, $2.2 billion, and $361.0 million, respectively, in the first half of 2018.
Sept. 18 2018, Updated 7:31 a.m. ET
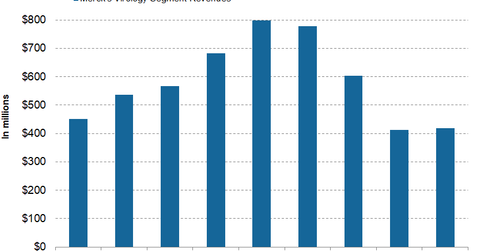
Merck’s Virology segment update
In the second quarter, Merck’s (MRK) Zepatier and Isentress reported revenues of $113.0 million and $305.0 million, respectively, which represented an ~78.0% decline and 8.0% growth on a YoY (year-over-year) basis. In the first half, Zepatier and Isentress reported net revenues of $243.0 million and $586.0 million, respectively.
Delstrigo approval
In August, the FDA approved Delstrigo, a combination of doravirine, lamivudine, and tenofovir disoproxil fumarate in fixed doses for the treatment of individuals with HIV-1 infection.
The FDA approval of Delstrigo was based on the data from the Phase 3 DRIVE-AHEAD trial, in which Delstrigo demonstrated consistent viral suppression over 48 weeks. In this trial, the drug achieved the primary endpoint of non-inferior efficacy compared to the efavirenz -600mg, emtricitabine-200mg, and tenofovir disoproxil fumarate 300mg combination regimen.
Pifeltro approval
In August, the FDA also approved Merck’s Pipeltro (doravirine), a novel NNRTI (non-nucleoside reverse transcriptase) for anti-HIV-1 therapy. The FDA recommended administration of Pipeltro in combination with other antiretroviral therapy
The FDA’s approval of Pipeltro was based on data from the Phase 3 DRIVE-FORWARD trial. In Phase 3 DRIVE-FORWARD trial, Pipeltro demonstrated consistent viral suppression over 48 weeks. During this timeframe, it achieved its primary endpoint of non-inferiority in efficacy compared to the darunavir 800 mg and ritonavir 100 mg combination regimen.
To learn more about the two Phase 3 clinical trials and clinical trial results, please refer to Merck’s August 30 press release.
Some important multi-class combination drugs for the treatment of HIV include Gilead Sciences’ (GILD) Atripla, Stribild, and Genvoya, as well as ViiV Healthcare’s Triumeq and Juluca. ViiV Healthcare is a joint venture between Pfizer (PFE) and GlaxoSmithKline (GSK) exclusively focused on the development of anti-HIV drugs.
Gilead Sciences’ Atripla, Genvoya, and Stribild generated revenues of $663.0 million, $2.2 billion, and $361.0 million, respectively, in the first half of 2018.
