Novavax's Manufacturing Problems Impact Global Vaccination Drive
Novavax stock was trading sharply lower on Oct. 20 due to manufacturing problems with its COVID-19 vaccine candidate.
Oct. 20 2021, Published 11:08 a.m. ET
Novavax stock was trading sharply lower on Oct. 20 due to reports of manufacturing problems with its COVID-19 vaccine candidate. What troubles is Novavax facing? What do the issues mean for the stock and the global vaccination drive?
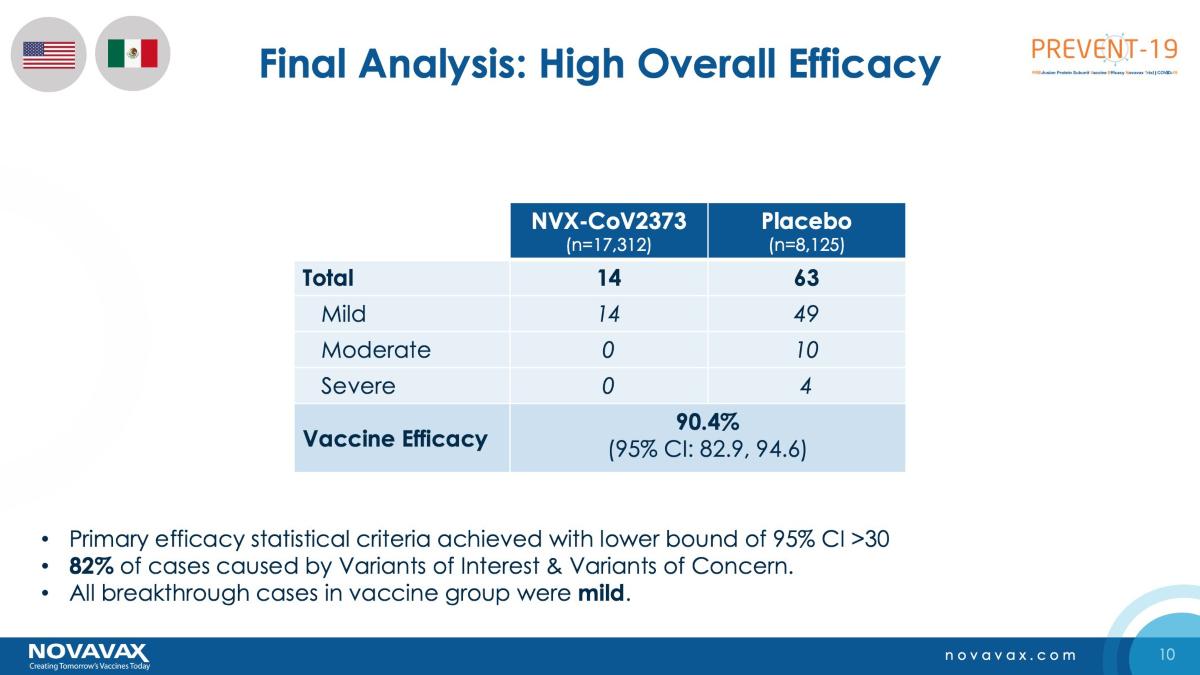
Novavax stock is up 42 percent YTD but has tumbled sharply from its 52-week highs of $331.68. The stock soared on Jan. 28 after the company reported that it completed the Phase 3 trials in the U.K., which showed an 89.3 percent efficacy. Now, with reports of manufacturing troubles, the stock has fallen to the levels where it traded before the announcement.
Politico on Novavax manufacturing problems
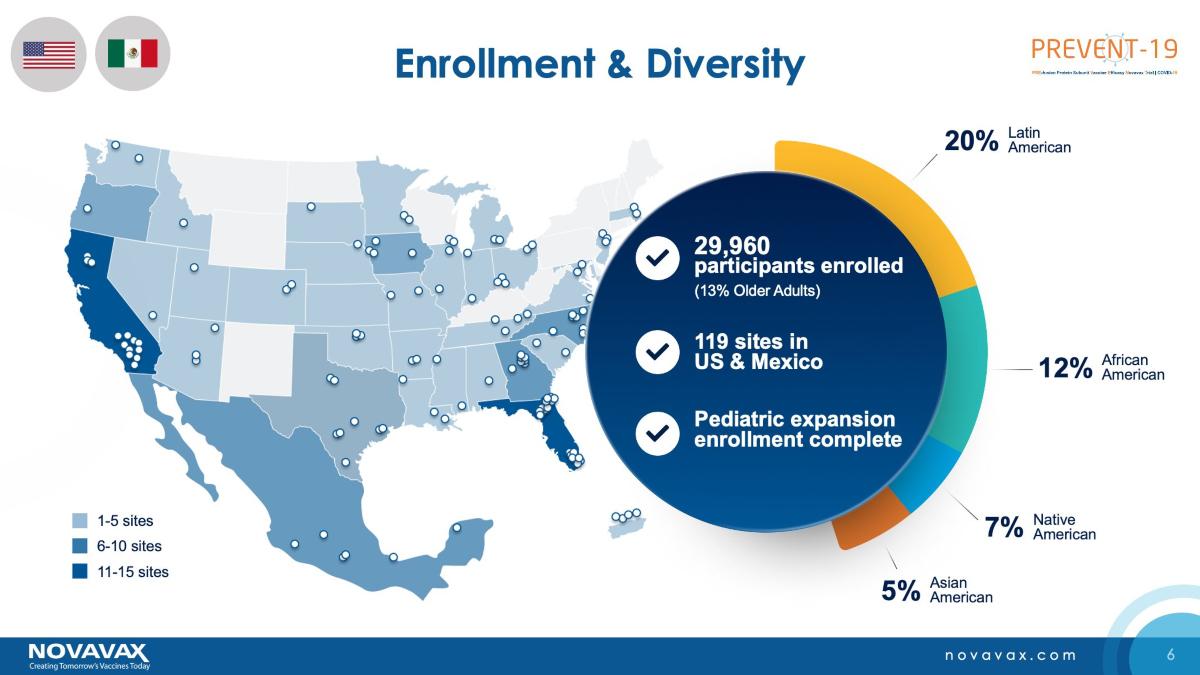
In 2020, the U.S. invested $1.6 billion in Novavax when the Trump administration went into overdrive on efforts to produce a COVID-19 vaccine. The investment in Novavax was the largest that the country made in a vaccine maker. However, Novavax has been running into recurrent production issues and also had to scale down its 2021 production target from 2 billion doses to 1.425 billion.
Reporting on Novavax’s production issues based on inputs from multiple unnamed sources, Politico said, “The methods it used to test the purity of the vaccine have fallen short of regulators’ standards and the company has not been able to prove that it can produce a shot that is consistently up to snuff.”
Politico said that the purity level for the Novavax vaccine is around 70 percent, which is below the 90 percent purity per batch that the FDA reportedly wants.
Is Novavax trying to get things done in a hurry?
While it takes years if not decades to make a new vaccine, there was a global scramble to make a COVID-19 vaccine for the worst health crisis that the world has faced in a century. It's worth noting that unlike companies like Johnson & Johnson that have a long track record of developing vaccines and other medications, Novavax hasn’t ever successfully developed a vaccine.
There was always an execution risk associated with Novavax and the troubles reported by Politico only exemplify the execution problems. As a person with knowledge about Novavax’s troubles said, “They rushed the process.”
The person also said, “It’s hard to make. And they can’t make it.” Even Johnson & Johnson faced troubles and had to shut a plant run by contract manufacturer Emergent BioSolutions after reports of contamination in 15 million doses.
What do Novavax's troubles mean for the global vaccination drive?
The manufacturing woes at Novavax could be a big setback for the global vaccination drive, especially in poorer nations. Half of the 2 billion COVID-19 doses that the international consortium known as COVAX was looking to provide to poorer countries is at stake due to the manufacturing problems at Novavax.
However, the U.S. vaccination drive shouldn't be impacted much. The country already has a big pile of vaccines even though the pace of vaccination has slowed down amid vaccine hesitancy in some regions.
What do the manufacturing troubles mean for Novavax stock?
Novavax stock was trading in single digits before it got into developing COVID-19 vaccines. If the company doesn't execute on the manufacturing, the stock could fall more from these levels.
Novavax responded to Politico’s report and reaffirmed its ability to deliver high-quality vaccines. “We are confident that our vaccine will soon play a significant role in the global COVID-19 vaccine arsenal, differentiated by its potential to help address two major issues slowing the world’s ability to end the pandemic: global distribution challenges and vaccine hesitancy," said Novavax CEO Stanley C. Erck.
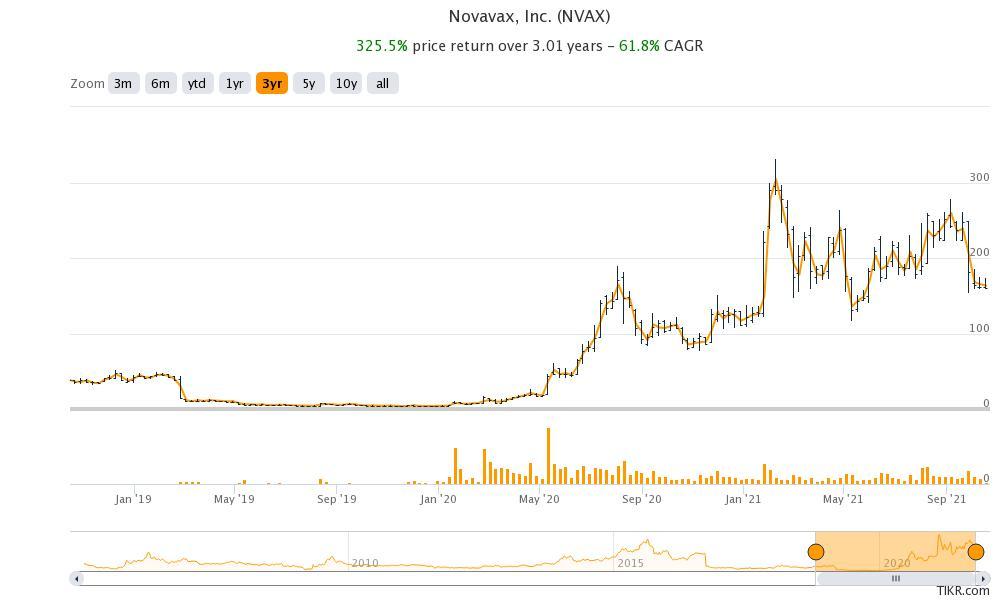
However, the company didn't comment on the doubts that the Politico report raised about the manufacturing problems. Markets don’t seem satisfied with Novavax’s response. The stock was trading lower in early price action despite the company denying any manufacturing problems.