A Look at Abraxane’s Revenue Growth Trajectory in 2018
In its third-quarter earnings conference call, Celgene (CELG) reiterated its expectation for Abraxane’s fiscal 2018 net product sales of around $1.0 billion.
Nov. 29 2018, Updated 10:30 a.m. ET
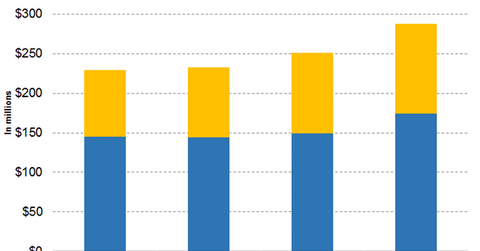
Abraxane revenue growth
In its third-quarter earnings conference call, Celgene (CELG) reiterated its expectation for Abraxane’s fiscal 2018 net product sales of around $1.0 billion. In the first nine months of 2018, Abraxane reported worldwide sales of $793 million, which is a YoY rise of 7.02%. Out of these revenues, $485 million were earned from the US market, which is a YoY rise of 7.30%. International markets contributed $308 million, a YoY rise of 6.57%.
In the third quarter, Abraxane reported worldwide sales of $288 million, which is YoY growth of 14.74%. Out of these revenues, $174 million came from the US market, which is a YoY rise of 16.78%. International markets contributed $114 million, which is a YoY rise of 11.76%.
Growth drivers
According to Celgene’s third-quarter earnings conference call, the company is awaiting results from phase 3 trial Apact, which is evaluating Abraxane in the pancreatic cancer indication.
On May 29, 2018, Roche Holdings (RHHBY) issued a press release announcing favorable results from phase 3 trial IMpower130, evaluating the combination of its immunotherapy Tecentriq with Abraxane in first-line metastatic in the non-squamous non-small cell lung cancer indication.
On June 2, 2018, Roche Holdings issued a press release announcing positive efficacy results from phase 3 trial IMpower131, evaluating the combination of Tecentriq with Abraxane in first-line advanced squamous NSCLC indication.
On July 2, 2018, Roche Holdings issued a press release announcing favorable results from the phase 3 trial IMpassion130, evaluating the combination of Tecentriq with Abraxane in the metastatic triple negative breast cancer indication.
These label expansion initiatives are expected to drive revenue growth for Abraxane in future years.
Fedratinib drivers
Celgene is working on submitting a new drug application (new drug application) for its investigational therapy, fedratinib, in myelofibrosis (or MDS) indication based on results from JAKARTA-1 and JAKARTA-2 trials by the end of 2018. According to Celgene’s third-quarter earnings conference call, there is only one FDA-approved therapy for MDS, and thus there remains significant unmet demand in this area.
