Electrophysiology Business May Be a Solid Growth Driver for BSX
In the first quarter, Boston Scientific (BSX) reported sales of close to $75 million in its electrophysiology business.
July 13 2018, Updated 9:02 a.m. ET
New product launches
In the first quarter, Boston Scientific (BSX) reported sales of close to $75 million in its electrophysiology business, reflecting a YoY (year-over-year) rise of ~17.2%. This rise was attributable to the rapid adoption of the company’s RHYTHMIA HDx mapping and navigation platform across multiple markets in the world.
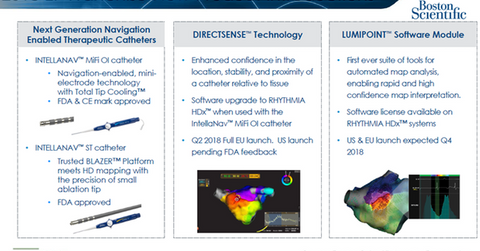
The RHYTHMIA mapping and navigation platform enables 3D high-density, high-resolution cardiac mapping to diagnose and treat arrhythmias during catheter ablation procedures. The company has also secured FDA approval for multiple next-generation navigation-enabled (or NAV) catheters to work in combination with RHYTHMIA for the ablation of arrhythmia. Boston Scientific plans to secure a CE mark for its force-sensing catheter, INTELLANAV STABLEPOINT, in 2019.
Additionally, Boston Scientific has introduced its software upgrade DirectSense technology to work in combination with its RHYTHMIA HDx hardware, its 2.0 RHYTHMIA software, and its IntellaNAV MiFi OI catheter. This technology is expected to witness a full launch in the European Union in the second quarter and to secure FDA approval in 2018.
Boston Scientific also plans to launch its third-generation RHYTHMIA mapping software, LUMIPOINT software module, in the United States and the European Union in the fourth quarter.
Product launches due to acquisitions
On October 2, 2017, Boston Scientific announced its acquisition of Apama Medical in a deal that added the Apama single-shot RF balloon to its portfolio. The company expects to secure a CE mark in the European Union and a clinical investigational device exemption (or IDE) for the device in the United States in the first half of 2019.
On April 3, Boston Scientific announced its acquisition of Securus Medical Group, a deal that added esophageal temperature monitoring technology to its portfolio. This technology is used to prevent thermal injury during ablation procedures. The company expects to launch the technology in the United States and the European Union in 2019.
