Pfizer’s Bosulif Had a Strong Performance in 4Q17 and 2017
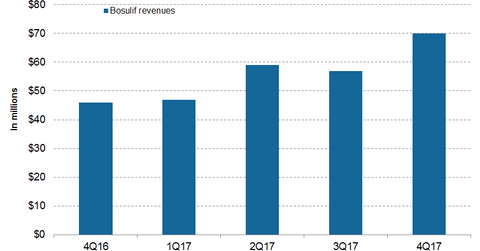
In 4Q17, Pfizer’s (PFE) Bosulif generated revenues of $70 million, which reflected ~52% growth on a YoY (year-over-year) basis.
Feb. 12 2018, Updated 2:10 p.m. ET
Bosulif revenue trends
In 4Q17, Pfizer’s (PFE) Bosulif generated revenues of $70 million, which reflected ~52% growth on a YoY (year-over-year) basis.
In 4Q17, in the US and international markets, Bosulif reported revenues of $46 million and $24 million, respectively—compared to $31 million and $15 million in 4Q16, which reflects ~50% and 55% growth on a YoY basis.
In fiscal 2017, Bosulif reported revenues of $233 million, which is ~39% growth on a YoY basis. In 2017, in the US and international markets, Bosulif generated revenues of $156 million and $77 million, respectively—compared to $115 million and $52 million in 2016.
In developed European markets and emerging markets in 2017, Bosulif reported revenues of $40 million and $5 million, respectively—compared to $28 million and $2 million in 4Q16.
Recent developments
In December 2017, the FDA approved Pfizer’s sBLA (supplemental new drug application) to expand the use of Bosulif to treat adults with newly-diagnosed chronic phase Philadelphia chromosome-positive CML (chronic myelogenous leukemia).
The FDA approval for Bosulif’s label expansion was based on data from the phase 3 BFORE trial. In the phase 3 BFORE trial, a significantly higher rate of patients on Bosulif achieved major molecular response (or MMR) at 12 months—compared to patients on imatinib.
At 12 months, patients on Bosulif had a CCyR (complete cytogenic response) rate of 77.2%—compared to 66.4% for patients on imatinib.
In September 2012, Bosilif received FDA approval to treat adults with chronic accelerated Philadelphia chromosome-positive CML.
Bosulif’s label expansion in December 2017 is expected to boost the drug’s sales in 2018.
In the CML drugs market, Bosulif’s peers include Takeda Pharmaceuticals’ (TKPYY) Iclusig, Bristol-Myers Squibb’s (BMY) Sprycel, and Novartis’s (NVS) Tasigna.
