FDA Proposes to Make OTC Hearing Aids Accessible—Who Will Sell Them?
The FDA proposed a rule that would allow the sale of over-the-counter hearing aids to make the devices easier to buy.
Oct. 20 2021, Published 2:19 p.m. ET

Hearing aids amplify sound and benefit people with hearing issues. If you struggle with hearing loss, you generally can purchase hearing aids after undergoing a medical exam, which sometimes requires a fitting with an audiologist.
With medical costs soaring, making it impossible for some to afford the visit(s) needed to purchase hearing aids, the FDA has proposed a rule to make over-the-counter hearing aids accessible. What companies will be offering over-the-counter hearing aids, and how soon can they be purchased?
What is the FDA proposing in its over-the-counter hearing aid rule?

The FDA is looking to establish a new category, specifically for over-the-counter hearing aids, and to amend “the regulatory framework for hearing aids.” This includes repealing the current conditions that are set for selling and purchasing these devices.
If approved, the rule would give “millions of Americans” access to over-the-counter hearing aids at a more affordable price, according to an FDA press release. The rule was also created to help “increase competition in the market.” The FDA’s proposed rule addresses President Joe Biden’s executive order on promoting competition in the U.S. economy.
How many people would the FDA’s over-the-counter hearing aid rule help?
Data shows that nearly “37.4 million American adults 18 and over struggle with hearing,” but only about “one-fifth of people who could benefit from a hearing aid use one.” Hearing aids are expensive devices, often ranging between $399 and $3,000. With this new proposed rule, individuals requiring the use of hearing aids might be able to afford them when purchasing the device over the counter.
To ensure that hearing aid users or those in need of the device are protected from being injured by “overamplification of sound,” the FDA’s proposed rule includes a volume limit for OTC hearing aids.
What companies will sell over-the-counter hearing aids?
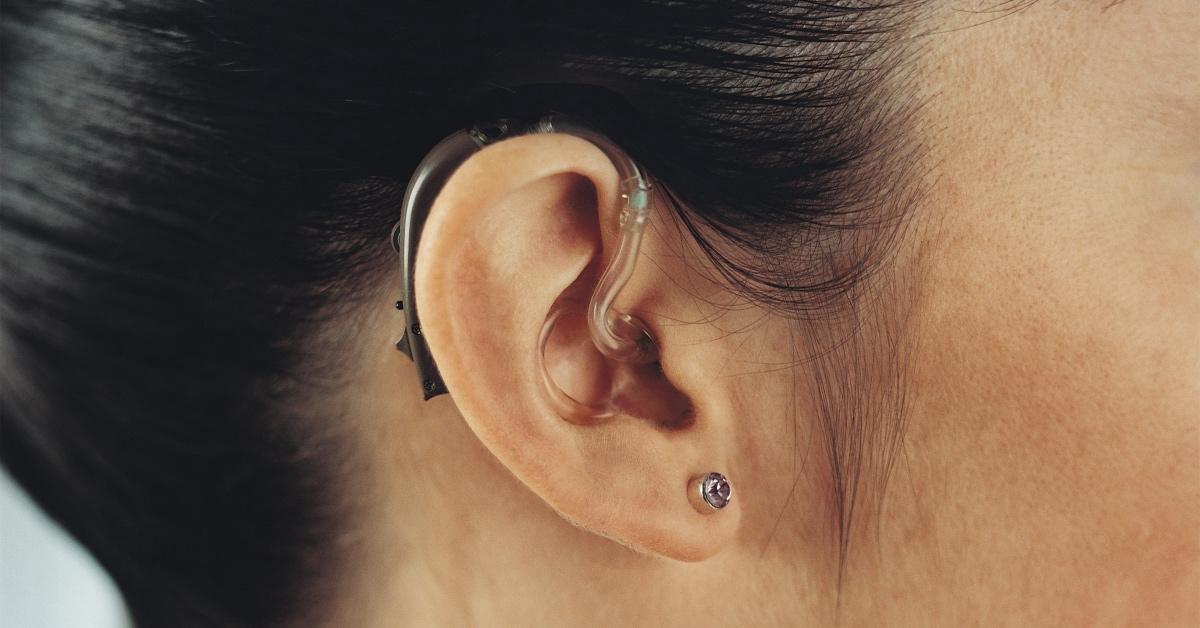
The FDA stated that over-the-counter hearing aids will be sold in brick-and-mortar retail stores and online rather than purchasing them directly from a doctor or a store that specializes in hearing aid sales.
Bose, Audien Hearing, and Eargo Store are a few retailers that currently sell hearing aids. They require patients to submit medical documentation before closing a sale.
Any hearing aids sold over-the-counter will be required to comply with any new regulations issued by the FDA along with those outlined in section 709 of the FDA Reauthorization Act of 2017 (FDARA). Physical and online stores that currently sell PSAPs (personal sound amplification products) might soon be able to market their devices as OTC as long as they comply with the FDA’s regulations.
PSAPs deliver a similar experience that someone would have if they were to wear a hearing aid. However, the FDA doesn't classify these devices as hearing aids.
FDA’s OTC hearing aid rule provides hope to those who cannot access or afford hearing aids
“Hearing loss has a profound impact on daily communication, social interaction, and the overall health and quality of life for millions of Americans,” according to Dr. Janet Woodcock, the acting FDA commissioner.
With the passing of this rule, millions of people will have access to a selection of hearing aids, offered at a more affordable price and without having to schedule a visit with their doctor.
While it isn’t clear when over-the-counter hearing aids will be available without needing a prescription or fitting, the public is open to comment on the proposed rule up until January 18, 2022.
