Tesaro Expects These Key Research Pipeline Milestones in 2019
As per Tesaro’s (TSRO) third-quarter earnings conference call, the company is anticipating results from Phase 1/2 trial, AVANOVA.
Dec. 13 2018, Updated 9:00 a.m. ET
Pipeline milestones
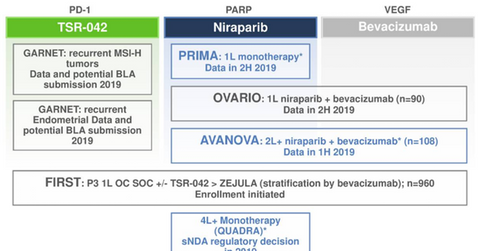
As per Tesaro’s (TSRO) third-quarter earnings conference call, the company is anticipating results from its Phase 1/2 trial, AVANOVA, which is being conducted in collaboration with ENGOT in the first half of 2019. In this trial, the company is comparing chemotherapy-free regimen Zejula (niraparib) monotherapy with the combination regimen of Zejula and Avastin (bevacizumab) in the recurrent ovarian cancer indication.
As per the company’s third-quarter earnings conference call, Tesaro is also expecting data from Phase 3 trial OVARIO evaluating the combination of Zejula and Avastin in treatment-naïve ovarian cancer patients in late 2019. Finally, as per the company’s third-quarter earnings conference call, Tesaro is also comparing chemotherapy with or without bevacizumab versus chemotherapy and Zejula maintenance therapy versus chemotherapy and Dostarlimab (TSR-042) therapy, in an ongoing Phase 3 trial, FIRST, in the first-line ovarian cancer indication.
Dostarlimab growth trends
In addition to Zejula, Tesaro is also focused on advancing its anti-PD-1 antibody, TSR-042 or Dostarlimab, in gynecologic tumors such as endometrial cancer and ovarian cancer. As per Tesaro’s investor presentation, endometrial cancer offers a market opportunity worth more than $1.0 billion for TSR-042 in the US and EU markets. According to Custom Study by Kantar Health based on SEER, endometrial cancer accounts for almost 93% of the total uterine cancers and affects almost 55,000 patients in the US annually.
As per Tesaro’s third-quarter investor presentation, the company is expecting data from GARNET study evaluating TSR-042 monotherapy in endometrial cancer patients with MSI-high status in 2019. After that, the company has also planned to file a biologics license application to seek FDA approval for TSR-042 in this indication at the end of 2019. As per the company’s third-quarter earnings conference call, MSI-high patients account for almost 25% of the total endometrial cancer patients.
