What to Expect from Roche’s Investigational Drug Polatuzumab Vedotin
In December 2017, Roche (RHHBY) presented the results of its randomized phase two GO29365 trial.
Jan. 10 2018, Updated 4:40 p.m. ET
Polatuzumab Vedotin
In December 2017, Roche (RHHBY) presented the results of its randomized phase two GO29365 trial. The trial compared Polatuzumab Vedotin in combination with bendamustine and Rituxan versus bendamustine and Rituxan alone in individuals with relapsed or refractory diffuse large B-cell lymphoma (or DLBCL) who are not subjects for a hematopoietic stem cell transplant.
Phase two clinical trial outcomes
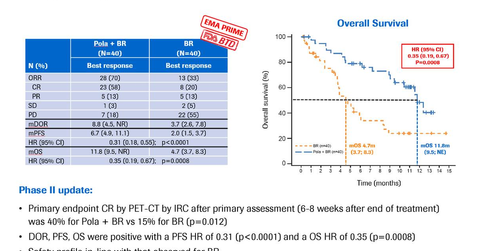
In the phase two GO29365 trial, polatuzumab vedotin along with bendamustine and Rituxan demonstrated an increased complete response (or CR) of 40% compared to 15% for bendamustine and Rituxan alone at the completion of the treatment. Patients on polatuzumab vedotin, bendamustine, and Rituxan combination therapy demonstrated an objective response (or OR) of 70% compared to 32.5% for patients on bendamustine and Rituxan alone.
Patients receiving polatuzumab vedotin, bendamustine, and Rituxan combination therapy demonstrated overall survival (or OS) of 11.8 months compared to 4.7 months for patients receiving bendamustine and Rituxan alone. Also, patients on polatuzumab vedotin bendamustine and Rituxan combination therapy showed a median progression-free survival of 6.7 months compared to 2.0 months for patients on bendamustine and Rituxan alone.
In the trial, patients receiving polatuzumab vedotin, bendamustine, and Rituxan combination therapy demonstrated the time to first response to treatment and disease worsening or duration of response of 8.8 months compared to 3.7 months for patients on bendamustine and Rituxan alone.
The success of the clinical trial could help Roche file a new drug application (or NDA) to the US FDA (Food and Drug Administration) and strengthen the company’s product portfolio. Presently, the drugs used to treat DLBCL include Vincristine, Pfizer’s (PFE) Adriamycin, Teva Pharmaceuticals’ (TEVA) Treanda, Roche’s Rituxan, and Bristol-Myers Squibb’s (BMY) Etopophos.
The Vanguard FTSE Developed Markets ETF (VEA) invests ~2.1% of its total portfolio holdings in Roche.
