How Did Keytruda Perform in 1Q17?
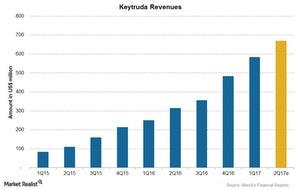
Merck (MRK) launched Keytruda in 4Q14 and reported global sales of $584 million for 1Q17. As a result, the company reported ~134% growth in revenues compared to $249 million in 1Q16.
Jul. 3 2017, Updated 10:37 a.m. ET
Keytruda
Keytruda is a prescription medicine classified under Merck & Co.’s (MRK) Immuno-oncology franchise. Keytruda is approved for various forms of tumors such as advanced non-small cell lung cancer, advanced melanoma, squamous cell carcinoma of head and neck, classical Hodgkin lymphoma, and advanced urothelial bladder cancer.
Merck (MRK) launched Keytruda in 4Q14 and reported global sales of $584 million for 1Q17. As a result, the company reported ~134% growth in revenues compared to $249 million in 1Q16. For 2Q17, revenues from Keytruda are expected to reach ~$672 million.
Importance of Keytruda
Keytruda is used for the treatment of advanced melanoma where the drug ipilimumab did not work, and the tumor shows an abnormal BRAF gene but the BRAF inhibitor did not work.
Keytruda is also used for the treatment of PD-L1 positive non-small cell lung cancer (or NSCLC) where the disease has progressed after platinum-based chemotherapy, and the tumor has an abnormal EGFR or ALK gene, but EGFR or ALK inhibitors did not work.
Keytruda is also approved for the treatment of recurrent or metastatic squamous cell carcinoma of head and neck (or HNSCC) for patients after platinum-based chemotherapy.
Keytruda is approved for classical Hodgkin lymphoma for patients who have received three or more treatments. It is also approved for patients who have undergone treatment, but the treatment did not work.
Keytruda is also approved for the treatment of advanced urothelial carcinoma, a type of bladder and urinary tract cancer, in patients who have undergone platinum-based chemotherapy.
Keytruda has also been approved for the microsatellite instability-high tumor or mismatch repair deficient solid tumors. This treatment would be appropriate for previously treated patients where the tumor has spread and cannot be removed by surgery.
Developments in treatment programs
Keytruda is under clinical development program studies for more than 30 tumor types in over 150 clinical studies. These studies include monotherapy as well as treatment in combination with other existing therapies.
In the next article, we’ll discuss the recent developments of Keytruda during June 2017.
For broad-based industry exposure, investors can consider the PowerShares Dynamic Pharmaceuticals ETF (PJP) which holds 5.1% of its total assets in Merck. PJP also holds 4.9% of its total assets in Pfizer (PFE), 4.6% in Ligand Pharmaceuticals (LGND), and 4.0% in Eli Lilly & Co. (LLY).
