Why the Patent Cliff Is a Key Driver of Generic Drug Growth
Over the last several years, patent cliffs have led to steep revenue losses for traditional pharmaceuticals as well as created a gateway for smaller companies to come to market with generics.
Sep. 1 2020, Updated 9:43 a.m. ET
Over the last several years, patent cliffs have led to steep revenue losses for traditional pharmaceuticals as well as created a gateway for smaller companies to come to market with generics. The case of Pfizer and Lipitor exemplified the consequences a traditional pharmaceutical company can suffer in the case of a patent expiration. In 2011 Pfizer lost exclusivity for its anti-cholesterol drug, Lipitor. Generic versions quickly emerged and, by 2014, sales of generic copies handily surpassed Lipitor sales.
It did not end with Pfizer. Novartis and Merck had similar experiences after the expiration of patents for Diovan (high blood pressure medication) and Singulair (asthma medication), respectively, in 2012. The list goes on. Looking forward, more than 150 drugs are expected to lose their patents over the next ten years, jeopardizing more than $190 billion in brand name drug sales.[1. “Biosimilars in Focus as Easy-to-Copy Large-Drug Expirations Wane.”] Bloomberg Industry Report, November 19, 2015. This presents a significant opportunity for generic drug manufacturers.

Market Realist
According to a report by DCAT, based on IMS analysis, the following are some of the key patent expiries between 2014 and 2018.
- Nexium (esomeprazole): This patent expired in May 2014. Innovator AstraZeneca granted a license to Teva (TEVA) to produce the generic form in the United States.
- Celebrex (celecoxib): This patent expired in 2014. Pfizer conceded the drug to generic drug makers Actavis (ACT) and Teva after a prolonged lawsuit.
- Symbicort (budesonide/formoterol fumarate dihydrate): Some of AstraZeneca’s 13 patents have expired, but all won’t expire until 2023. No generic version of the drug exists.
- Crestor (rosuvastatin): AstraZeneca will lose its patent protection for Crestor in 2016.
- Cialis (tadalafi): Eli Lilly (LLY) is set to lose patent protection in the United States and Europe in 2017.
According to data from IMS, small-molecule products worth $121 million will lose patent protection in developed markets between 2014 and 2018. The IMS also forecasts that $48 million worth of biologic products will lose patent protection over the next three years (Source: DCAT).
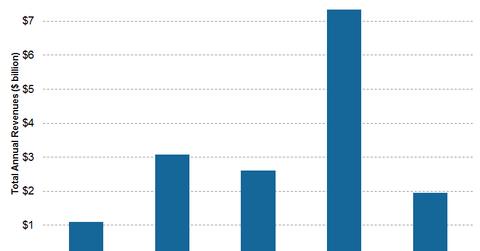
AstraZeneca (AZN) will lose patent protection on two major drugs in 2016: Crestor and Seroquel XR. Together, the pair accounts for annual revenues of $7.3 billion. The graph above shows companies that will lose patent protection on their blockbuster drugs in 2016 and the revenues these drugs have pulled in annually.

According to estimates by IMS, generics (GNRX) will constitute 52% of global pharmaceutical expenditure growth. Branded drugs will constitute only 35% of growth. IMS also estimates that generic drug revenues will climb to $442 billion in 2018 from $267 billion in 2014.
Investors looking to foray into the generic drugs market could look at the VanEck Vectors Generic Drugs ETF (GNRX), which seeks to replicate the price and yield performance of the Indxx Global Generics and New Pharma Index. The graph above shows the ETF’s major holdings.
Generic drugs offer a unique opportunity for long-term and medium-term investors.
Definitions:
The Hatch-Waxman Act is the informal name given to the Drug Price Competition and Patent Term Restoration Act of 1984. One of the main provisions of the Act is that it allows for an expedited FDA approval process for generic drugs while also granting certain market and patent exclusivity for both branded and generic drug companies.
A patent cliff refers to a situation when one or more of a company’s products’ patent protections expire. The expiration exposes the company’s product to external competition and potential significant loss of revenue.
